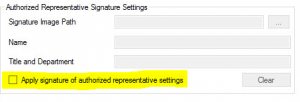
The ONVIF test tools have a built-in feature that enables you to upload an electronic signature of an authorized representative and apply it to your product’s Declaration of Conformance (DoC) document. With the submission of a DoC with an electronic signature and all other necessary documents, the approval process will be automatic and your product will be immediately listed on the Conformant Products page of the ONVIF website.
To apply an electronic signature to the DoC, follow steps 1-2 below before running conformance tests: